Endodontic Microsurgery Tools 2025–2029: Breakthroughs Set to Transform Dental Care Revealed!
Table of Contents
- Executive Summary: Key Insights for 2025 and Beyond
- Market Size and Growth Forecasts Through 2029
- Breakthrough Technologies Driving Innovation
- Major Players and Company Strategies
- Regulatory Landscape and Compliance Updates
- Emerging Trends in Microsurgical Techniques
- Regional Analysis: Growth Hotspots and Opportunities
- Challenges and Barriers to Adoption
- Sustainability and Sterilization Innovations
- Future Outlook: What’s Next for Endodontic Microsurgery Instrumentation?
- Sources & References
Executive Summary: Key Insights for 2025 and Beyond
The field of endodontic microsurgery instrumentation is poised for notable advancements and expansion in 2025 and the following years. Driven by the increasing adoption of minimally invasive dental procedures, practitioners and clinics are investing in advanced tools designed to improve surgical outcomes, ergonomics, and patient recovery times. Key players are emphasizing precision, durability, and the integration of digital technologies to streamline microsurgical workflows.
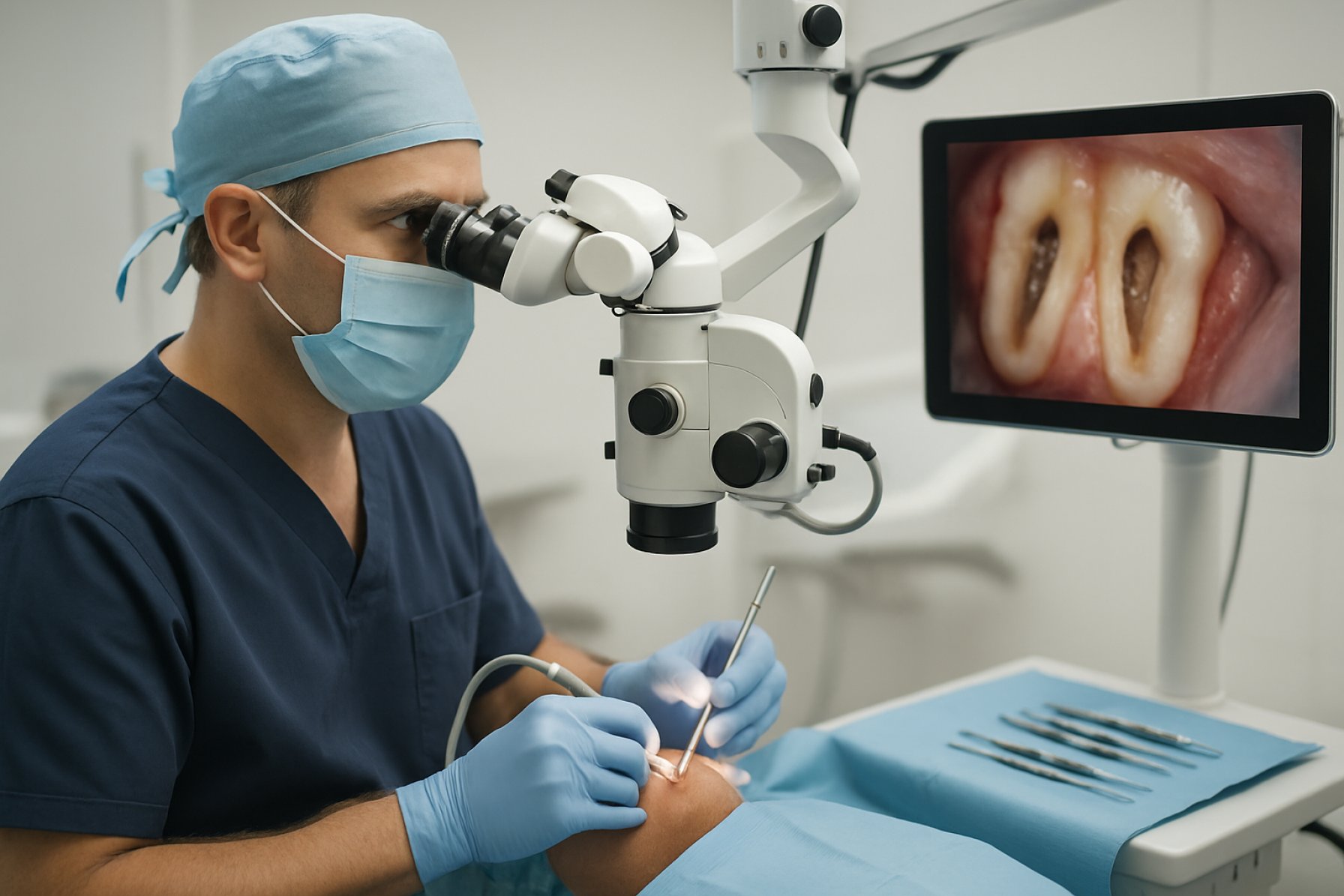
A significant trend shaping the sector is the widespread use of dental operating microscopes, which now offer enhanced magnification, illumination, and integrated digital imaging capabilities. Leading manufacturers such as Carl Zeiss Meditec AG and Leica Microsystems are continuously refining their platforms to offer better ergonomics and workflow integration, responding to practitioner demand for precision and ease of use.
Microsurgical instrument sets are also evolving, with companies like Dentsply Sirona and Kerr Dental introducing titanium and stainless steel micro-instruments optimized for root-end management, retrograde filling, and tissue preservation. These developments are accompanied by a growing emphasis on single-use, pre-sterilized instruments to enhance infection control and procedural efficiency, aligning with evolving clinical guidelines and patient safety standards.
Ultrasonic surgical units, vital for root-end preparation during endodontic microsurgery, are being redesigned for improved power delivery, noise reduction, and integration with irrigation systems. For instance, ACTEON Group and Satelec (a part of ACTEON) are advancing piezoelectric devices with refined tip geometries and user-friendly interfaces, supporting greater adoption among dental professionals.
Looking ahead, digitalization will further influence the market, with 3D navigation tools and augmented reality likely to become more prevalent in microsurgical training and intraoperative guidance. Industry leaders are investing in digital workflow solutions that integrate imaging, planning, and instrument tracking to boost accuracy and procedural confidence.
Overall, the outlook for endodontic microsurgery instrumentation in 2025 and beyond is characterized by rapid technological progress, increasing global adoption, and a focus on enhancing clinical outcomes. Manufacturers are expected to continue prioritizing innovation, safety, and digital integration, meeting the evolving needs of dental professionals and patients alike.
Market Size and Growth Forecasts Through 2029
The market for endodontic microsurgery instrumentation is experiencing robust growth in 2025, driven by advances in dental technology, increasing prevalence of apical periodontitis, and the rising demand for minimally invasive endodontic procedures. Key instruments in this sector include microsurgical handpieces, ultrasonic tips, micro mirrors, micro forceps, surgical operating microscopes, and associated consumables, all tailored for precise and effective root-end surgeries. Manufacturers are continually innovating to enhance ergonomics, visualization, and cutting efficiency, which is sustaining market momentum.
Recent data from leading industry players highlight an uptick in global adoption rates. For instance, Dentsply Sirona and Kerr Dental report increased sales of microsurgical kits and digital solutions across North America, Europe, and Asia-Pacific. The expansion of dental clinics and specialty endodontic practices in emerging economies is further fueling the demand for advanced instrumentation. Global Surgical Corporation, renowned for its surgical microscopes, has noted rising orders from both private and institutional customers, citing the growing emphasis on treatment success rates and patient outcomes.
Market analysts within the sector anticipate a compound annual growth rate (CAGR) in the mid-to-high single digits through 2029. The proliferation of continuing education programs and hands-on training—often supported by manufacturers such as Carl Zeiss Meditec and Leica Microsystems—is expected to accelerate instrument adoption among dental professionals. Additionally, improvements in material science, particularly with the development of high-strength alloys and enhanced tip coatings, are extending the lifespan and efficacy of microsurgical tools, which is a key purchasing consideration for clinics.
Looking ahead, the global endodontic microsurgery instrumentation market is forecasted to reach new highs by 2029, underpinned by technological integration such as digital workflow solutions and image-guided surgery. The growing focus on patient comfort, surgical precision, and predictable healing will likely drive further innovation and purchasing activity, especially in regions investing in advanced dental infrastructure. Key players are expected to continue diversifying their product lines and expanding educational initiatives to support market growth through the remainder of the decade.
Breakthrough Technologies Driving Innovation
The landscape of endodontic microsurgery instrumentation in 2025 is shaped by rapid technological advancement, precision engineering, and integration of digital solutions. Noteworthy innovations are centered on improving visualization, ergonomics, and minimally invasive techniques, all of which aim to enhance clinical outcomes and patient comfort.
One of the most significant breakthroughs is the adoption of high-definition, 3D digital microscopes. These devices—such as the PROergo from Carl Zeiss Meditec AG—enable endodontists to perform microsurgery with enhanced depth perception and magnification. The transition from optical to digital visualization supports real-time collaboration, remote consultation, and improved documentation. The integration of augmented reality overlays is also being explored, providing procedural guidance directly in the clinician’s field of view.
Microsurgical instruments themselves are becoming increasingly refined. Manufacturers like Dentsply Sirona and Kerr Corporation are introducing microsurgical kits featuring ultra-thin, ergonomically contoured instruments with advanced surface coatings to reduce tissue trauma and improve tactile feedback. New materials, including titanium alloys and specialized ceramics, are being employed for increased durability and biocompatibility.
Another notable trend is the integration of ultrasonic technology into microsurgical instruments. Systems such as the Variosurgical from NSK-Nakanishi Inc. offer precise bone cutting and root-end preparation with minimal collateral damage, enabling more predictable surgical outcomes. The adoption of piezoelectric devices is anticipated to expand further, driven by clinical studies confirming their efficacy in delicate endodontic procedures.
Digital workflow integration is a defining theme moving forward. Companies like Hu-Friedy are developing microsurgical instrument sets compatible with digital planning systems, allowing endodontists to pre-plan incision and flap designs based on 3D CBCT imaging. The next few years are likely to see further convergence of digital imaging, 3D printing (for custom surgical guides), and smart instrument tracking for enhanced procedural accuracy.
Looking ahead, the sector is poised for continued innovation, with an emphasis on user-centered design, minimally invasive approaches, and synergy between digital and mechanical technologies. These advancements are expected to set new standards for precision, safety, and efficiency in endodontic microsurgery instrumentation by 2026 and beyond.
Major Players and Company Strategies
The endodontic microsurgery instrumentation sector has witnessed dynamic shifts in recent years, driven by technological innovation, strategic partnerships, and targeted product development from major dental manufacturers. As of 2025, established leaders such as Dentsply Sirona, Kerr Dental (a subsidiary of Envista Holdings), and COLTENE are actively shaping the market through the introduction of advanced microsurgical kits and devices tailored for precision, ergonomics, and minimally invasive procedures.
Dentsply Sirona, leveraging its robust R&D and global distribution network, continues to expand its surgical endodontics portfolio. In 2024, the company introduced updates to its surgical ultrasonic systems and micro-instrument lines, emphasizing improved tactile feedback and enhanced visualization for clinicians. Their strategic focus remains on integrating digital workflows with microsurgical instrumentation, supporting both training and clinical outcomes (Dentsply Sirona).
Kerr Dental has focused on developing endodontic microsurgery kits that feature ultra-fine micro-mirrors, micro-pluggers, and micro-scalpels, as well as specialized root-end filling instruments. The company’s emphasis is on delivering ergonomically optimized and corrosion-resistant tools. In recent years, Kerr has also strengthened its educational initiatives, partnering with key opinion leaders to promote best practices in microsurgical endodontics (Kerr Dental).
COLTENE has advanced its MicroMega line, introducing single-use microsurgical instruments and proprietary surface coatings to improve both precision and infection control. Their 2025 strategy includes collaborating with dental schools and research institutes to pilot new tool designs and integrate clinician feedback into product refinement cycles (COLTENE).
Other notable players include Brasseler USA, which continues to innovate in surgical burs and ultrasonic tips, and Obtura Spartan Endodontics (Microsurgery Dental), recognized for specialty root-end microsurgery kits. These companies are increasingly investing in digital integration—such as 3D navigation aids and enhanced visualization systems—reflecting the broader trend toward precision-guided endodontic microsurgery.
Looking forward, the competitive landscape is expected to intensify as digital dentistry, AI-driven imaging, and single-use microsurgical instruments gain ground. Strategic alliances, clinician training programs, and R&D investments in ergonomic design will remain central to the growth strategies of leading manufacturers in this evolving sector.
Regulatory Landscape and Compliance Updates
The regulatory landscape for endodontic microsurgery instrumentation is experiencing notable developments in 2025, influenced by both technological advancements and evolving international standards for medical devices. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are placing increased emphasis on the safety, efficacy, and traceability of precision dental instruments, impacting how manufacturers design, validate, and market endodontic microsurgery tools.
In the United States, the FDA continues to regulate endodontic microsurgery instruments under the Medical Device Amendments, classifying most microsurgical tools as Class I or II devices depending on their specific application. In 2024 and into 2025, the FDA has intensified requirements for Unique Device Identification (UDI) and post-market surveillance, aiming to improve traceability and patient safety. This affects manufacturers such as Dentsply Sirona and Kerr Corporation, who must ensure that their instruments comply with updated labelling, documentation, and adverse event reporting protocols.
In the European Union, the Medical Device Regulation (MDR 2017/745) became fully applicable in May 2021 and continues to shape the market through the transition period ending in 2028. Manufacturers supplying the EU, including global leaders like Coltene and MicroMega, are adapting to stricter requirements for clinical evidence, technical documentation, and post-market surveillance. These include increased scrutiny of reusable surgical instruments, enhanced vigilance systems, and additional demands for clinical performance data relevant to endodontic microsurgery.
Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) is also updating its guidelines to align more closely with international standards, notably in the areas of biocompatibility and sterilization validation, which are particularly relevant to microsurgical instruments (PMDA). Meanwhile, China’s National Medical Products Administration (NMPA) is accelerating its review pathways for innovative dental instruments and increasing the frequency of on-site audits of overseas manufacturers (NMPA).
Looking forward, industry observers expect more harmonization of global medical device regulations as organizations such as the International Organization for Standardization (ISO) revise technical standards for dental instruments. These updates are likely to focus on digital integration, cybersecurity (for connected devices), and more robust clinical data requirements, which will impact both established and emerging manufacturers of endodontic microsurgery instrumentation.
Emerging Trends in Microsurgical Techniques
Endodontic microsurgery instrumentation is experiencing rapid advancement, with several emerging trends shaping the field in 2025 and beyond. The integration of digital technology, improvements in instrument design, and the adoption of innovative materials are driving changes that aim to enhance precision, reduce patient morbidity, and improve long-term outcomes.
One of the most significant trends is the increased use of surgical operating microscopes with enhanced optical and ergonomic features. Manufacturers such as Carl Zeiss Meditec and Leica Microsystems have introduced models offering superior magnification, integrated digital imaging, and hands-free control options. These microscopes, combined with 3D visualization systems, are facilitating more accurate root-end resections and retrograde fillings.
Another key development is the refinement of ultrasonic microsurgical tips and handpieces. Companies like Dentsply Sirona and KaVo Dental are producing tips with diamond coatings and specialized angulations for improved access and efficacy during root-end preparations. The use of ultrasonics has been shown to minimize bone removal and reduce operative time, supporting faster patient recovery.
Micro-instruments such as micro-mirrors, micro-pliers, and micro-curettes are also being redesigned for greater durability and tactile sensitivity. HuFriedyGroup has released instrument lines with enhanced ergonomic handles and corrosion-resistant materials, aiming to reduce clinician fatigue and improve procedural accuracy.
The adoption of biocompatible and bioactive materials for retrograde fillings—such as mineral trioxide aggregate (MTA) and newer bioceramics—continues to expand. FKG Dentaire and Brasseler USA are providing application systems that enable precise placement of these materials, which promote superior sealing and tissue regeneration.
Looking ahead, the integration of digital workflow solutions—including guided microsurgery and 3D-printed surgical guides—is anticipated to become more prevalent. Companies like Planmeca are developing platforms that merge CBCT data with digital planning tools, streamlining complex procedures and reducing human error.
Overall, the outlook for endodontic microsurgery instrumentation points to continued innovation, with devices and materials tailored for minimally invasive, patient-centered care set to dominate the market in the next few years.
Regional Analysis: Growth Hotspots and Opportunities
The global market for endodontic microsurgery instrumentation is witnessing dynamic regional shifts, with significant growth hotspots emerging in North America, Europe, and Asia-Pacific. In 2025 and the coming years, these regions are poised to drive innovation, adoption, and market expansion, supported by differing factors such as technological advancements, demographics, and healthcare infrastructure.
North America continues to lead in the adoption of advanced endodontic microsurgery instrumentation. The United States, in particular, benefits from a dense network of dental specialists, advanced training programs, and high patient awareness. Companies such as Dentsply Sirona and Ultradent Products, Inc. maintain a strong presence, offering a comprehensive portfolio of microsurgical instruments and endodontic systems. The region’s regulatory environment, combined with robust reimbursement frameworks, further encourages the uptake of precision instruments in endodontic practice.
Europe remains a key growth region, driven by an increasing emphasis on minimally invasive dental procedures and favorable government support for oral health initiatives. Countries such as Germany, France, and the United Kingdom are seeing heightened demand for microsurgical instruments, supported by the presence of leading manufacturers like KaVo Dental and COLTENE. The European Federation of Endodontology’s ongoing educational programs are fostering skill development and expanding the clinical adoption of advanced microsurgical tools across the continent.
Asia-Pacific is emerging as the fastest-growing market for endodontic microsurgery instrumentation, propelled by expanding dental infrastructures and rising disposable incomes. China, India, South Korea, and Japan are central to this growth, with local and international manufacturers such as J. Morita Corporation and Mani, Inc. expanding their regional presence. Increasing investments in dental education and the proliferation of multispecialty clinics are expected to accelerate market expansion. The Asia Pacific Endodontic Confederation’s advocacy for advanced treatment standards is also contributing to the region’s rapid adoption of modern microsurgical instruments.
Looking ahead, the Middle East and Latin America are expected to present new opportunities, driven by improving healthcare access and government-led oral health campaigns. Manufacturers are strategically partnering with local distributors to tap into these emerging markets, offering tailored solutions to meet region-specific needs.
Overall, the outlook for endodontic microsurgery instrumentation points to robust regional growth, underpinned by continuous product innovation, expanding education, and evolving patient expectations across global markets.
Challenges and Barriers to Adoption
Endodontic microsurgery instrumentation has witnessed significant technological advancements, yet several challenges and barriers continue to impede widespread adoption as of 2025 and looking forward. A primary obstacle remains the high initial investment required for state-of-the-art surgical microscopes, ultrasonic tips, and specialized micro-instruments. Leading manufacturers such as Carl Zeiss Meditec and Leica Microsystems offer advanced surgical microscopes, but their cost can be prohibitive for many smaller practices, especially in developing markets.
Another persistent challenge is the steep learning curve associated with mastering microsurgical techniques and the corresponding instrumentation. Despite comprehensive training programs and workshops offered by organizations like the American Association of Endodontists and manufacturers such as Dentsply Sirona, practitioners often require extensive hands-on experience to achieve proficiency. This limits the number of clinicians able to perform endodontic microsurgery at a high level, constraining patient access to these advanced procedures.
Maintenance and sterilization of delicate microsurgical instruments present further hurdles. Microsurgical instruments and ultrasonic tips, such as those from Kerr Dental, require specialized care to maintain their function and longevity. Many practices face logistical and financial difficulties in adhering to the stringent protocols necessary for optimal instrument upkeep, increasing the risk of instrument failure or infection.
Reimbursement is another significant barrier. In numerous healthcare systems, the reimbursement rates for endodontic microsurgery do not reflect the higher costs and time commitments associated with using advanced instrumentation. This economic disincentive, highlighted by practitioners and noted by industry bodies such as the American Association of Endodontists, can deter investment in new technologies.
Looking ahead, manufacturers are addressing these challenges by developing more affordable, user-friendly, and durable instruments. For instance, Brasseler USA and Microsurgical Technology are pursuing innovations aimed at reducing complexity and improving cost-effectiveness. However, broad adoption will require ongoing collaboration between industry, professional associations, and regulatory bodies to address training, economic, and logistical barriers over the next several years.
Sustainability and Sterilization Innovations
In 2025, sustainability and sterilization innovations are central to the evolution of endodontic microsurgery instrumentation. Growing pressure from both regulatory bodies and environmentally conscious dental professionals is driving manufacturers to develop instruments and processes that reduce environmental impact while ensuring uncompromised sterility.
A major trend is the transition toward reusable microsurgical instruments with enhanced durability. Companies such as Dentsply Sirona and Kerr TotalCare are expanding their portfolios of stainless steel and titanium instruments that withstand repeated sterilization cycles without degradation, aiming to reduce the need for single-use tools. These instruments are increasingly packaged using recyclable or biodegradable materials, reflecting a broader industry shift toward sustainable packaging solutions.
Sterilization technology is also advancing rapidly. The integration of low-temperature hydrogen peroxide plasma and ozone-based sterilizers is gaining traction, as these systems consume less energy and water than traditional steam autoclaves and produce fewer chemical residues. Getinge and MELAG are among those introducing compact, high-throughput sterilizers specifically tailored for dental clinics, offering validated cycles for delicate microsurgical instruments. These new-generation sterilizers often include data logging and connectivity features, supporting compliance with global sterilization standards and digital traceability.
Instrument tracking and lifecycle management is another area of focus. RFID and laser marking technologies are being implemented to monitor instrument usage, automate sterilization workflows, and reduce unnecessary waste. For example, Integra LifeSciences provides solutions that allow clinics to follow each instrument’s journey, optimizing replacement schedules and minimizing environmental footprint.
Looking ahead, the emphasis on eco-design principles is expected to intensify. Manufacturers are exploring bio-based polymers for handles and packaging, as well as modular designs that allow for the replacement of worn components rather than discarding entire instruments. Moreover, the adoption of global sustainability frameworks such as the FDI World Dental Federation’s Sustainability in Dentistry initiative is anticipated to further accelerate green innovation and reporting across the sector.
As sustainability becomes a competitive differentiator, endodontic microsurgery instrumentation is set to benefit from ongoing investments in greener manufacturing, advanced sterilization, and circular economy practices well into the latter half of the decade.
Future Outlook: What’s Next for Endodontic Microsurgery Instrumentation?
As the field of endodontics advances through 2025 and beyond, the future of microsurgical instrumentation is poised for substantial innovation, shaped by ongoing technological refinement, heightened clinician expectations, and patient demand for minimally invasive, predictable outcomes. The integration of digital technologies, improvements in material science, and the proliferation of ergonomic, precision-engineered tools are set to define the next era in endodontic microsurgery.
One of the most notable trends is the expanding adoption of surgical microscopes enhanced with digital visualization and augmented reality overlays. Manufacturers such as Global Dental Microscopes are developing next-generation systems offering ultra-high-definition imaging, improved depth perception, and real-time documentation capabilities, facilitating more precise surgical navigation and enhanced patient communication. Advanced illumination and hands-free control features are also becoming standard, reducing procedure time and operator fatigue.
In parallel, ultrasonic surgical instruments continue to evolve. Companies like Dentsply Sirona are pioneering handpieces with enhanced power modulation, better tactile feedback, and finer tip geometries. These improvements enable clinicians to perform root-end preparations and retrograde fillings with greater conservatism and accuracy, preserving tooth structure and supporting faster healing.
Material innovation is another key driver, particularly with the increasing use of bioceramic-based root-end filling materials and micro-instruments crafted from advanced alloys. For example, Kerr Dental is investing in premium microsurgical kits that incorporate corrosion-resistant metals and ergonomic handles to optimize control and longevity. The push toward single-use, pre-sterilized instruments—cited by Hu-Friedy Group—responds both to infection control priorities and workflow efficiency.
Looking forward, artificial intelligence (AI) and robotics are attracting significant R&D attention, with companies like Zeiss collaborating on AI-powered image analysis for intraoperative guidance and post-surgical assessment. The integration of digital workflow tools, such as guided endodontic surgery planning software and 3D-printed surgical guides, is anticipated to become increasingly routine, enabling greater predictability in complex cases.
In sum, the next few years will likely see endodontic microsurgery instrumentation characterized by further digitalization, smarter materials, and enhanced ergonomics—all converging to support the goals of precision, safety, and patient comfort. Continuous collaboration between manufacturers and clinicians will remain essential to ensure new tools meet the evolving demands of contemporary endodontic practice.